Balancing the nitrogen cycle

Nitrogen is fundamental to life on our planet, being one of the primary nutrients essential for all living organisms. Despite gaseous N2 making up around 78% of the volume of the Earth’s atmosphere it is largely inaccessible in this form for most organisms and must be converted into reactive forms. This blog discusses the importance of nitrogen to Earth’s ecosystem, and outlines how human activities are shifting the balance of the nitrogen cycle, with potentially dire consequences. It also delves into the benefits of stable isotope analysis, highlighting how it can be used to generate powerful data to better understand the migration of nitrate through the global nitrogen cycle.
The global nitrogen cycle
The methods by which nitrogen moves and transforms through the Earth’s ecological systems – atmosphere, biosphere, hydrosphere, and geosphere – is known as the global nitrogen cycle, which is fundamental to sustaining life on our planet. This natural cycle is driven by processes such as nitrogen fixation, nitrification and denitrification, which involve the conversion of nitrogen between various chemical forms that support life and maintain ecological balance. For example, a select group of organisms convert N2 from the vast pool of gas present in the atmosphere into reactive forms – such as ammonia – which is subsequently harnessed by plants and other organisms to grow and thrive. Gruber and Galloway depicted this global nitrogen cycle – including all the major processes that transform molecular nitrogen into reactive nitrogen and back again – in their 2008 Nature paper (Figure 1),1 which also clearly highlighted the immense mass of reactive nitrogen species that human activities add to this fragile balance.
Unbalancing the nitrogen cycle
A growing global population and ever-increasing demand for food production has resulted in various human activities that have caused a massive acceleration of the natural reactive nitrogen cycle. These actions have approximately doubled the input of reactive nitrogen into the Earth’s ecosystem, with the fertilizers used to help feed the planet’s population accounting for approximately half this total. This has pushed our global nitrogen cycle beyond what is deemed safe by many experts in the field,2 with concentrations of reactive forms of nitrogen – nitrate in particular – increasingly observed not only in surface waters, but also in groundwater.
Dr Ben Surridge, biogeochemist and senior lecturer at Lancaster University in the UK, explained: “In many locations around the world, groundwater has effectively become a stock of reactive nitrogen species that are gradually being released to surface water. Unfortunately, regardless of current corrective strategies at the land surface, this is likely to continue for decades to come. For example, increases in nitrate concentration in the River Thames have been seen for more than 140 years,3 with rises in the early 1970s thought to have been the result of delayed delivery through the groundwater system from increases in nitrate accumulation decades earlier. These results are supported by other research that shows increases in nitrate concentration occurring in chalk, limestone and sandstone aquifers across the UK, with similar challenges facing other countries around the world.4,5”
Consequences of this disruption
The impacts of human disturbances to the reactive nitrogen cycle are far reaching, ranging from a loss of biodiversity to soil acidification, and can even directly affect human health. Ben continued: “Perhaps the most significant consequences arise from eutrophication; excessive plant or algal growth resulting from the accumulation of reactive nitrogen (or other naturally limiting growth factors) in various ecosystems. Algal blooms are perhaps the most widely reported indicator of this phenomenon, and can affect fresh water or marine ecosystems, posing a risk to wildlife, pets, livestock and, in some cases, humans.”
The blue-green algae bloom in Lough Neagh – the British Isles’ largest lake – in 2023 was a prime example of the devastation caused by eutrophication, which was attributed, in part, to nutrient influx from agriculture.6

One of the key consequences of algal blooms is hypoxia – low levels of dissolved oxygen in a water body – which has become especially notable in coastal waters, as well as in some lakes. Ben added: “The number of known hypoxic coastal areas has increased exponentially over the past six decades, reaching more than 500 sites and covering over a quarter of a million square kilometers.7 These ‘dead zones’ pose a significant risk to aquatic ecosystems, including the loss of marine life and biodiversity. One high profile example is the dead zone in the Gulf of Mexico. This phenomenon has been observed since the 1980s, and results from nitrogen and phosphorus being exported into the Mississippi River and, eventually, the coastal region. Disturbance to the nitrogen cycle in fresh waters can also lead to acidification, damaging aquatic ecosystems and resulting in a decrease of natural biodiversity.”
Impacts on human health
There is still much debate on the impacts of reactive nitrogen species, particularly nitrate, on human health. Various associations have been suggested – including to various cancers – but perhaps the highest profile of these is the potential link between nitrate and infant methemoglobinemia, a life-threatening blood disorder. These concerns have led to strict limits on nitrate in drinking water.
Knowledge is key
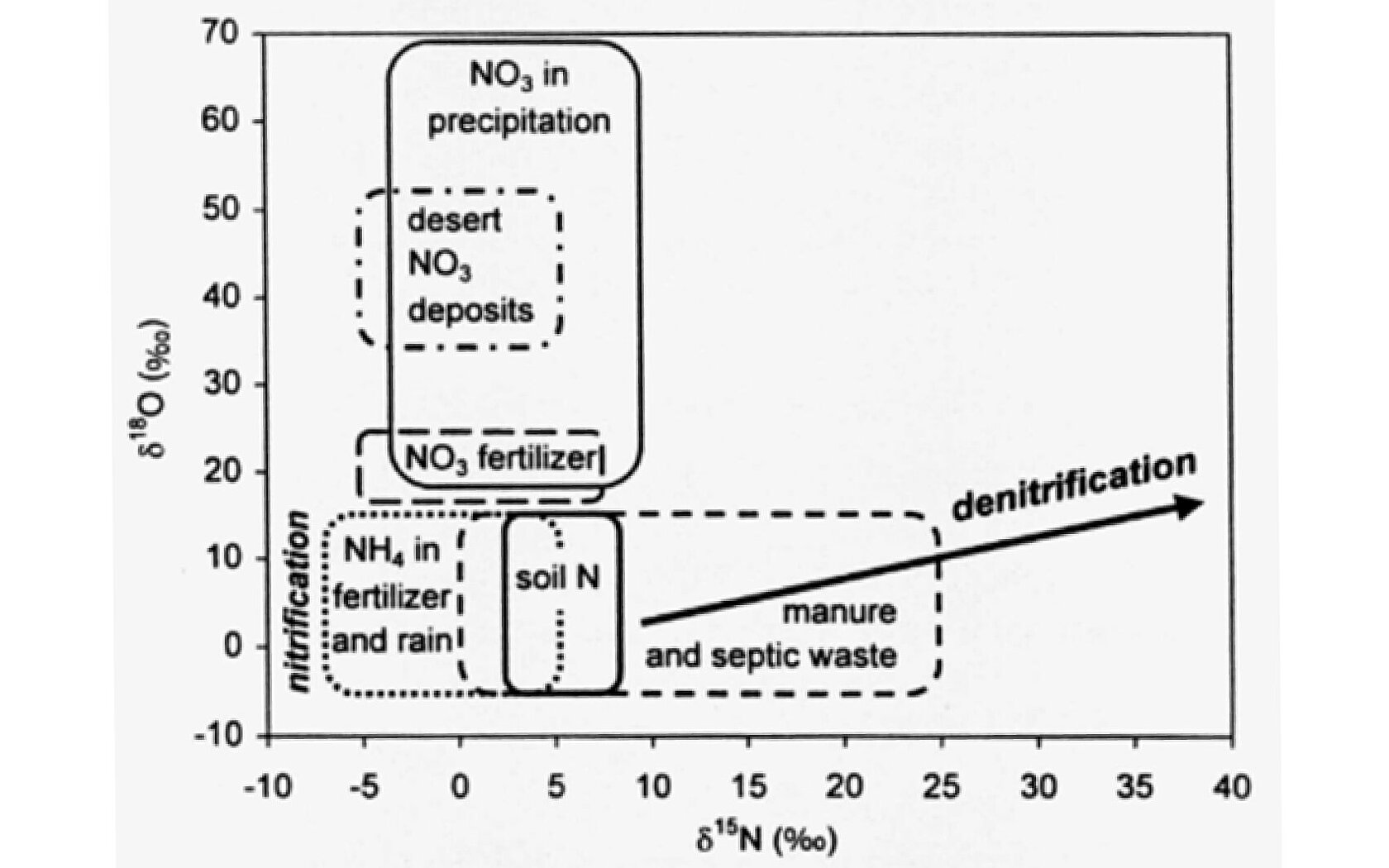
Efforts to rebalance the nitrogen cycle are therefore important to mitigate these issues and promote a healthy and sustainable ecosystem. This can only be achieved if we know the individual sources of reactive nitrogen in ecosystems and understand their impacts. Stable isotope analysis for nitrate pollution is often the first step to garner these insights. Since the 1970s, stable isotope analysis of nitrate has been possible, but the technique became more mainstream when Dr Carol Kendall developed a simple dual isotope plot in the late 1990s (Figure 2). That plot allowed researchers to quickly identify and characterize the probable sources of nitrate in environmental samples, as explained in detail in our blog, Celebrating 25 Years of Dr Carol Kendall’s Dual Isotope Plot of Nitrate Sources. The data used to generate this plot were derived from global sources, but it’s important to look at local sources of contamination to be able to implement changes at a regional or more local level. This highlights the need for rapid and cost-effective approaches to undertake dual isotope analysis, which can help to address many of the challenges discussed above.
Dual stable isotope data for nitrate can be generated using instruments like our EnvirovisION platform, which can process large numbers of environmental samples. These data can be used to help pinpoint areas where nitrate is an issue and, together with the Kendall plot, inform work to identify potential sources of nitrate in these areas. These techniques can also provide data to help with modelling future scenarios and designing and implementing strategies to try to bring the nitrogen cycle back into balance.
References
- Gruber N, Galloway J. An Earth-system perspective of the global nitrogen cycle. Nature. 451, 293–296 (2008). https://doi.org/10.1038/nature06592
- Richardson K, Steffen W, Lucht W, et al. Earth beyond six of nine planetary boundaries. Sci Adv. 2023;9(37). doi:10.1126/SCIADV.ADH2458
- Howden NJK, Burt TP, Worrall F, et al. Nitrate pollution in intensively farmed regions: What are the prospects for sustaining high-quality groundwater? Water Resour Res. 2011;47(11). doi:10.1029/2011WR010843
- Wang L, Stuart ME, Lewis MA, et al. The changing trend in nitrate concentrations in major aquifers due to historical nitrate loading from agricultural land across England and Wales from 1925 to 2150. Science of the Total Environment, The. 2016;542:694-705. doi:10.1016/j.scitotenv.2015.10.127
- DeSimone LA, McMahon PB, Rosen MR. The quality of our Nation’s waters: Water quality in principal aquifers of the United States, 1991-2010. Circular. Published online 2015. doi:10.3133/CIR1360
- EUMETSAT. (2023) Lough Neagh algal bloom. https://user.eumetsat.int/resources/case-studies/loch-neagh-algal-bloom. Accessed July 31, 2024.
- Dai M, Zhao Y, Chai F, et al. Persistent eutrophication and hypoxia in the coastal ocean. Cambridge Prisms: Coastal Futures. 2023;1:e19. doi:10.1017/CFT.2023.7
- Kendall C, McDonnell JJ. Isotope Tracers in Catchment Hydrology. Elsevier; 1998.
Find Out More
Learn more about nitrate isotope analysis in our whitepaper. We spoke to Dr. Leonard Wassenaar about his simplified method for nitrate isotope analysis, the titanium (III) method, which dramatically simplifies the sample preparation process.

DOWNLOAD YOUR COPY
Fill in the form to receive your download link by e-mail.
By clicking on the "Subscribe" button, I consent to the receipt of personalized newsletters via e-mail by Elementar Analysensysteme GmbH and its group companies companies as well as the evaluation of my user behavior in this regard and - if available - the merging of this data with my data in our customer database. In order to receive newsletters from our group companies, it is necessary to transfer your above-mentioned personal data to these companies. We point out that these are partly located in so-called unsafe third countries outside the EU/EEA , in which no adequate level of data protection (e.g. by adequacy decision of the EU, Art. 45 GDPR) is guaranteed. In these countries, you may not be able to enforce your rights as a data subject, or only to a limited extent. In addition, it is possible that local government agencies access your data to a disproportionate extent. The transfer of data to these recipients is therefore only legitimized by your consent pursuant to Art. 49 (1) lit. a) GDPR, which you grant with your subscription. The newsletter can be unsubscribed at any time with effect for the future as well as my consent to the third country transfer can be revoked at any time. A revocation does not affect the lawfulness of the processing carried out on the basis of the consent until the revocation. For further information, please refer to our privacy policy.
Do not miss any new articles
NEWSLETTER
We will constantly publish new blog articles. Register for our newsletter to stay up-to-date and get informed about latest blog articles, news and trends.

